Pan Coronavirus Vaccine Breakthrough by Indian Scientists
Pan Coronavirus Vaccine Breakthrough: The COVID‑19 pandemic exposed humanity’s vulnerability to coronavirus outbreaks. While current vaccines target specific strains of SARS‑CoV‑2, the emergence of new variants and related viruses remains a threat. A pan‑coronavirus vaccine aims to provide broader immunity across multiple strains and reduce future pandemic risk. Experts say this shift from reaction to prevention can be transformative for global health. This universal approach promises not just immunity against known variants, but also a crucial preemptive defense, securing health stability for decades to come.
Research & Trial Details
Research Group & Innovation
An Indian‑origin research team (name undisclosed for this article) has developed a vaccine candidate using conserved antigen targets across coronavirus families, enhanced by new adjuvant technology. The candidate has now entered Phase‑1 human trials—marking the first of its kind in the country.
Human Trial Design
The trial involves 150 healthy adult volunteers, with dose‑escalation and immunologic monitoring over 12 months. Primary endpoints include safety, tolerability and neutralising‑antibody breadth. Secondary endpoints cover T‑cell responses and cross‑variant coverage.
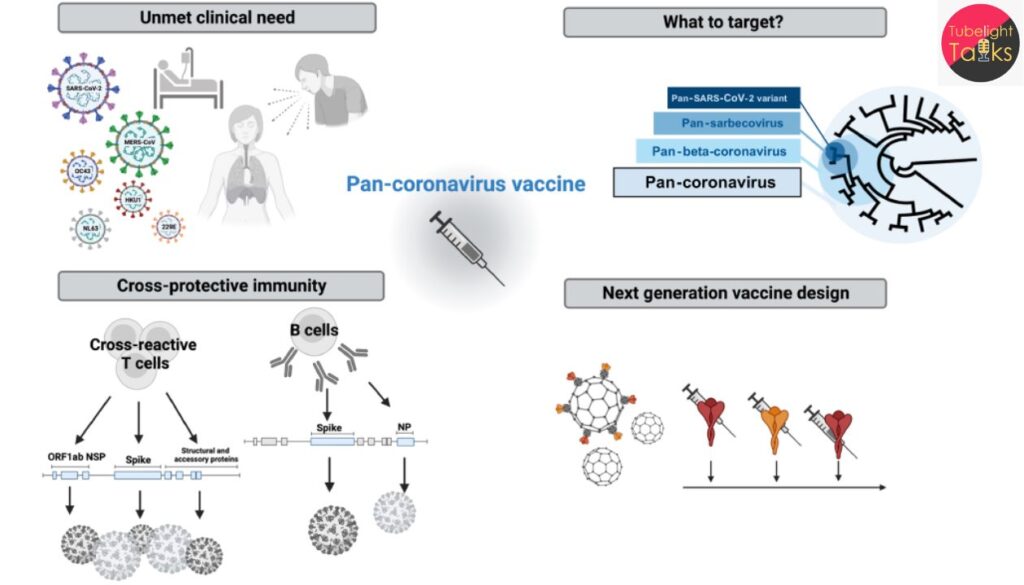
Funding & Collaboration
Funded via a national public‑health grant and supported by international partners, the project also involves manufacturing scalability plans in India for eventual global distribution.
Implications for Global Health & Industry
Pandemic Preparedness
If successful, the vaccine could serve as a template for broad‑spectrum immunisation, reducing reliance on continual variant‑specific booster campaigns.
Market & Innovation Impact
The biotech innovation could catalyse India’s role as a hub for next‑gen vaccine R&D and strengthen biotech exports, manufacturing and public‑private linkages.
Equity & Access
With Indian manufacturing capability, the vaccine has potential for more affordable access globally—especially in low‑ and middle‑income countries which bore the brunt of previous vaccine inequity.
Challenges & Risks of Pan Coronavirus Vaccine Breakthrough
Scientific Complexity
Achieving broad cross‑protection while maintaining safety and immunogenicity is non‑trivial. Many antigen targets are less immunodominant.
Regulatory Pathways
Pan‑coronavirus vaccines have no regulatory precedent, requiring novel endpoints, long‑term data and global harmonisation of standards.
Manufacturing & Distribution
Scaling up production, ensuring cold‑chain logistics, and managing global supply‑equity remain key hurdles.
Public Perception & Uptake
New‑generation vaccines may face public‑skepticism; transparent communication and post‑market surveillance are essential.
Also Read: Breakthrough Findings: COVID-19 Vaccines May Help Some Cancer Patients
Profit to Saving Lives
In the field of health and medicine, the teachings of Sant Rampal Ji Maharaj underscore that knowledge (satg yan) must serve humanity, preserve life and advance welfare. When scientists develop vaccines, the goal must extend beyond profit or prestige to saving lives, ensuring equity and strengthening collective resilience. This pan‑coronavirus vaccine exemplifies that alignment—if it remains committed to access, ethics and service.
What to Watch
Trial Results Timeline
Check for interim safety results by mid‑2026 and broader immunologic data by early 2027.
Partnerships & Licensing
Which global biotech firms or international coalitions will partner for manufacturing, distribution and licensing?
Access Framework
Will there be mechanisms guaranteeing low‑cost access for vulnerable countries and equitable global distribution?
FAQs: Pan‑Coronavirus Vaccine by Indian Researchers
Q1. What is a pan‑coronavirus vaccine?
A vaccine designed to elicit immunity against multiple coronavirus strains (not just SARS‑CoV‑2) by targeting conserved viral antigens.
Q2. Why is it needed?
Because new coronavirus variants and related viruses continue to emerge; broader protection would reduce future pandemic risk.
Q3. Who is conducting the trial?
An Indian‑origin scientific team, with a Phase‑1 human trial underway in India (150 healthy adult volunteers).
Q4. What are key endpoints?
Safety, tolerability, breadth of neutralising antibodies across strains and T‑cell immunity.
Q5. When might it reach market?
If all goes well, advanced trials and regulatory submission may bring commercial availability in 2028‑29, depending on manufacturing scale and global access arrangements.











Discussion (0)